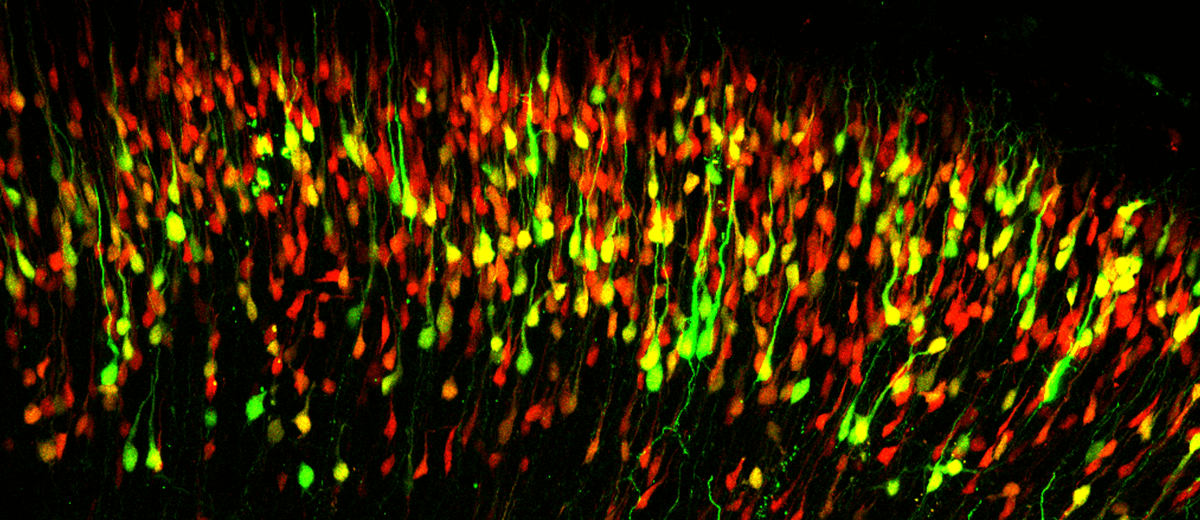
Molecular mechanisms regulating the generation of celular diversity in the neocortex
The neocortex is responsible for many higher-order functions including information processing and language. Defects in neocortical circuits are associated with many neurological disorders including schizophrenia, autism and mental retardation. Tightly regulated mechanisms are required to control cortical development from the generation of nerve cells to their assembly into circuits. Despite all the recent progress, many critical questions regarding cortical development remain unclear including the mechanisms used by cortical neural stem cells, known as radial glial cells (RGCs), to generate the cellular diversity in the cortex. The prevalent hypothesis proposes that neuronal fate is determined by the birthdate of these cells, suggesting the existence of a common RGC progenitor that becomes progressively restricted during development. More recent evidence provided by multiple independent laboratories suggest the existence of diversity in the pool of RGCs with the identification of RGC subpopulations pre-specified to generate upper layer cortico-cortical neurons (ULNS) and B1 cells.
We are currently working on the characterization of the molecular mechanisms regulating the different cellular behaviors of a subpopulation of RGCs specified to generate ULNs, using molecular biology and histological studies together with functional studies using in utero electroporation. Upper layer cortical projection neurons are critical for complex thought and higher associative tasks, and seem to be more susceptible to dysfunction in neuropsychiatric disorders, so the understanding of the mechanisms involved in its proper specification is of critical importance. We are also interested in the study of how alterations in the production of ULNs impacts mammalian behavior and relates with disease, taking advantage of conditional knockout mice for cell adhesion proteins and related molecules. These mice present increased cerebral cortex due to the overproliferation of radial glia cells. Such elarged cerebral cortex is related with an increase in the number of neurons expressing markers of ULNs.
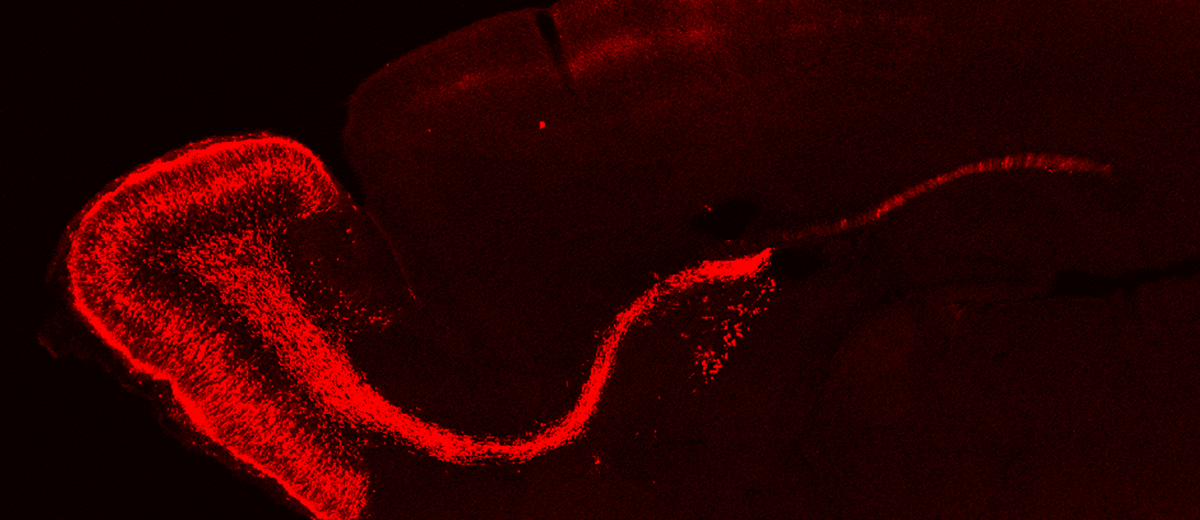
Activation dynamics of quiescent B1 cells along postnatal development
Adult neurogenesis occurs in two brain regions in mammals, the subependimal zone (SEZ) of the lateral ventricles and the subgranular zone of the dentate gyrus of the hippocampus. Adult neural stem cells (NSCs) of SEZ, also called B1 cells, generate neuroblasts, which migrate long distances through the rostral migratory pathway (RMS) until reaching the olfactory bulb (OB) where these cells differentiate into multiple types of interneurons. These NSCs also produce glia cells that include astrocytes and oligodendrocytes that integrate into regions near the production site. B1 cells retain an organization and morphology similar to radial glia cells, from which they descend, presenting a radial process linked to blood vessels and primary cilia in the apical part in contact with the cerebrospinal fluid. These cells are pre-specified during the embryonic period (between embryonic days 13 and 15 (E13-E15) remaining quiescent for long periods of time until they begin to reactivate at postnatal ages. Recently it has been described that most of the divisions of adult B1 cells in active state would be symmetric of two types: self-renewing (20-30%) or terminals producing two intermediate progenitors (80-70%). It is known that adult neurogenesis declines with age, however, the activation dynamics of the quiescent NSCs of the SVZ from the postnatal period until the adult age is unknown. These NSCs could follow different activation dynamics throughout the postnatal development until the adult state, which could imply differences in their progeny and type of division. To investigate this hypothesis, we are using the in utero and ex utero electroporation technique that allows specific labeling of neural cell subpopulations and their progeny and in vivo disturbance of genes of interest, combined with lineage tracing studies.
